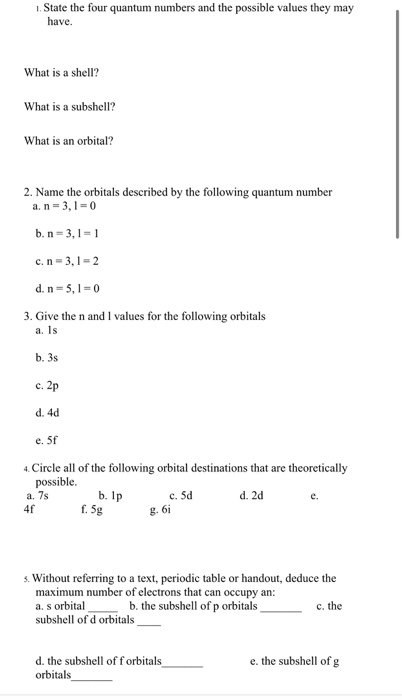
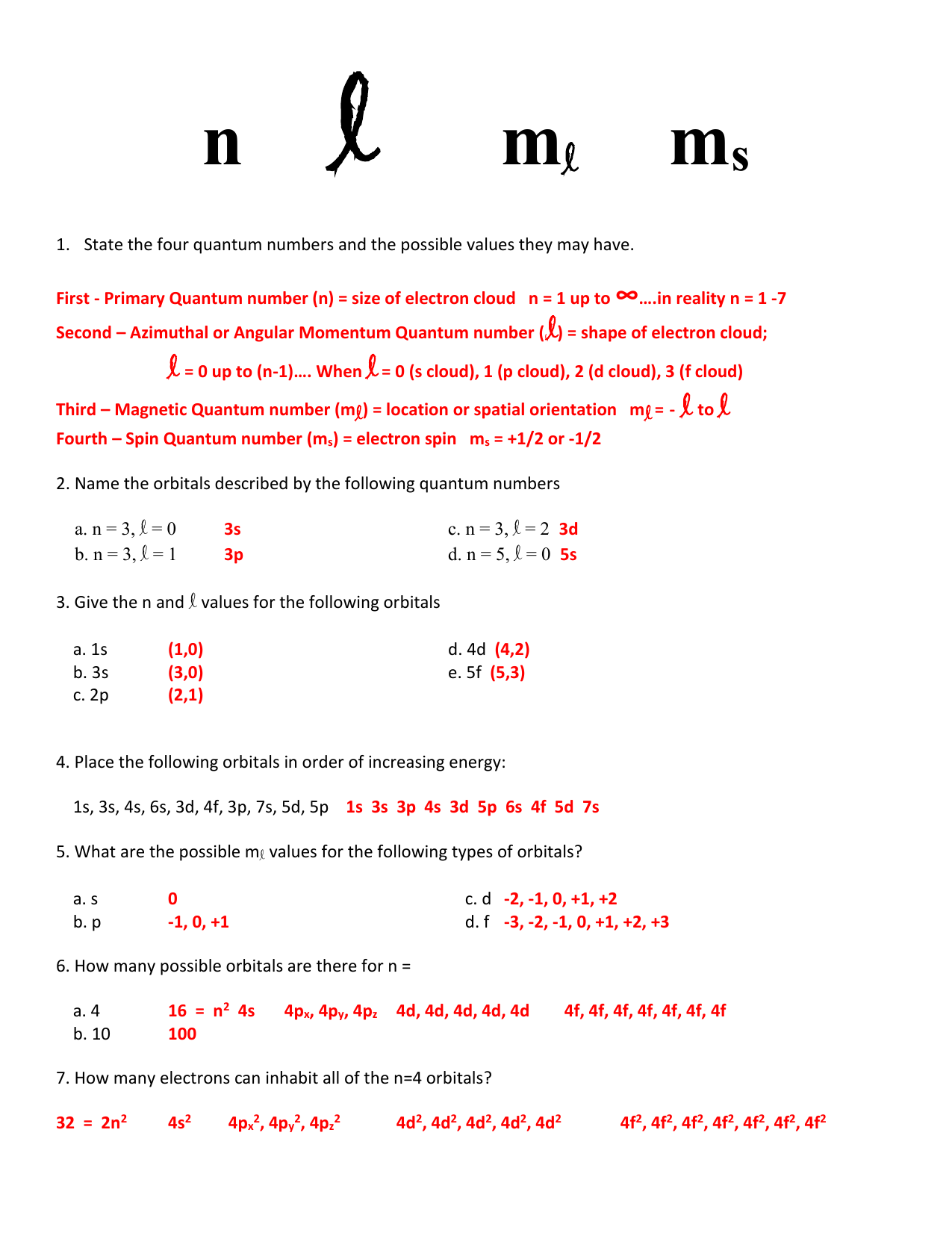
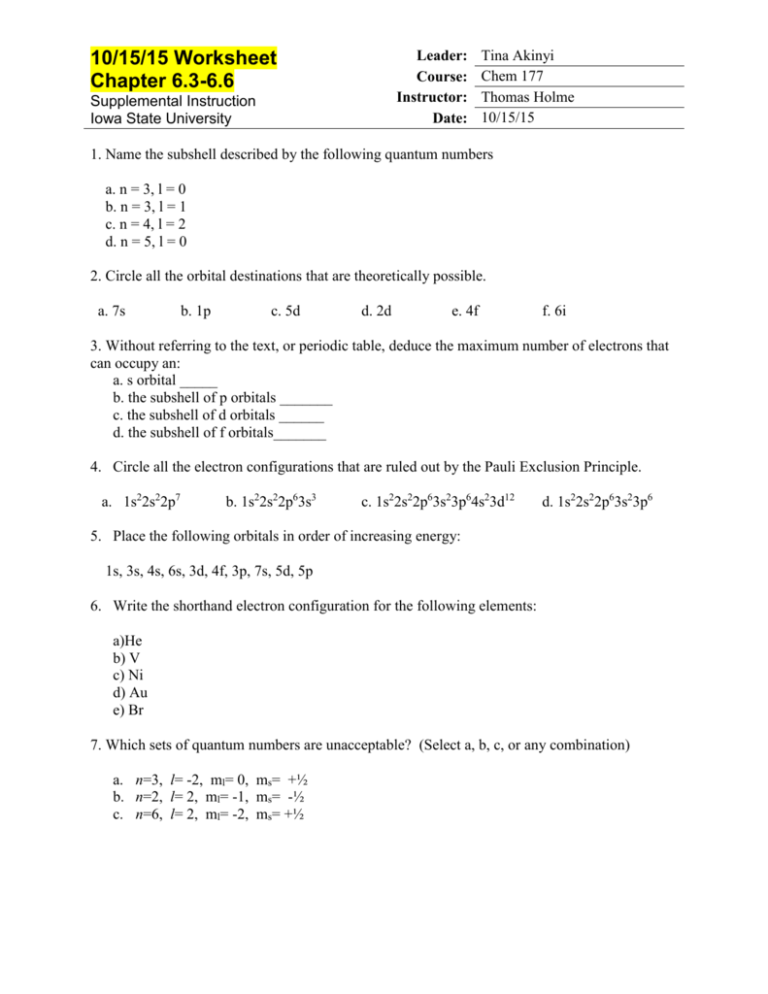
Name the Orbitals Described by the Following Quantum Numbers
The allowed values of Ifor the shell with 2 are e. The subshell with the quantum numbers r4 1-2 is c.
1s 3d 4s 6d 2p4f What are the possible m values for the following types of orbitals.

. Circle all of the following orbital destinations that are theoretically possible. The number of orbitals with the quantum numbers n3 1-2 and m 0 is b. M l is a range of l.
Give the n and l values for the following orbitals. The orbital with the following quantum numbers are given below. Finally for n 2 and l 1 you have the second energy level and the 2p-subshell.
The allowed values of 1 for the shell with n-4 are c. B n 3. A n1 l 0.
The number of orbitals with the quantum numbers n3 1 The subshell with the quantum numbers n4 12 is The ml values for a d orbital are The allowed values of I for the shell with n2 are The allowed values of I for the shell with n4 are The number of orbitals in a shell with n3 is The number of orbitals with n3 and 11 is 2 The maximum number of electrons with. Label the orbitals described by each of the following sets of quantum numbers. Thus d orbital corresponds to 4 double dumb-belled shapes d xy d yz d zx d x 2 y 2 with the atomic nucleus at its centre and one dumb belled with dough nut shaped d z 2.
In other words the magnetic quantum number identifies the specific orbital which holds the electron. 5f Place the following orbitals in order of increasing energy. Place the following orbitals in order of increasing energy.
SOLVEDName the orbitals described by the following quantum numbers and name one element in that orbital. 3p c 2s d. Ml 2 this is the 4dyz orbital.
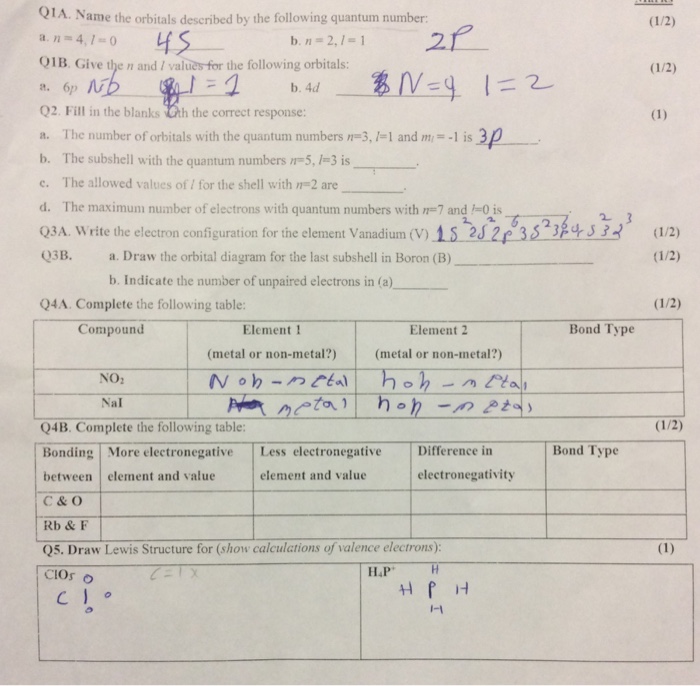
QUANTUM NUMBERS WORKSHEET Name Ras Muinidlsti 1. N 3 L 1 c. N 3 l b.
Give the n and l values for the following orbitals 2 0 a. Represents the electron and its spin. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital.
Name the orbitals described by the following quantum numbers a. Represents the number of orbits possible. Using s p d notations describe the orbital with the following quantum numbers.
N 3 l 0b. L is a range of n-1. For d orbital Azimuthal quantum number l 2 and the magnetic quantum number m -2 -1 0 1 2.
1s 3s 4s 6s 3d 4f 3p 7s 5d 5p 4. D orbital has two nodal planes. The number of orbitals in a shell with n3 is 2.
How many possible orbitals are there for n 3p 5s a 4 n 2 16 orbitals b 10 n 2 100 orbitals. See what the community says and unlock a badge. Name the orbitals described by the following quantum numbers a.
Name the orbitals described by the following quantum numbers n3 and l1 Explanation n is the principal quantum number if n 3 then it is 3 shell and if. The subshell with the quantum numbers n-5 1-3 is c. In a hypothetical universe the quantum numbers for an atomic orbital follow these rules.
L 1 m_l -1 0 1 By convention we take m_l 0 to represent the p_z orbital. The m values for a d orbital are d. Each electron in an atom is described by four different quantum numbers.
A n 3 l 2 b n 7 l 4 c n 5 l 1. Name the orbitals described by the following quantum number. The values of ml for this subshell are.
The allowed values of for the. Name the orbitals described by the following quantum number n3 l 0 Explanation n is the principal quantum number and l 0 designates for the s subshell it means that this orbital is. The number of orbitals with the quantum numbers n-31 1 and m-1 is 3p b.
Ml Magnetic quantum number. N 5 l 2. N 3 L 0 b.
N 3 l 2 d. N 3 l 1c. State the four quantum numbers and the possible values they may have.
N 3 L 2 d. N 5 0. In an atom the order of.
Circle all of the following orbital destinations that are theoretically possible. Therefore a total of five orbitals can share the quantum nubmers n 4 and l 2. Hence d orbitals have five orientations in space.
N 3 l 0 b. Name the orbitals described by the following quantum numbers a. Ml 0 this is the 4dxy orbital.
L Secondary Quantum NumberOrbital Shape Quantum number. Ms Spin Quantum number. N 3 l 1 c.
Name the orbitals described by the following quantum number S2. N any positive integer value from 2 to infinity l any positive integer value from 2 to n1 ml any. C n 4.
Give the n and L values for the following orbitals a. Ml 1 this is the 4dxz orbital. Name the orbitals described by the following quantum numbera.
Fill in the blanks Crh the correct response. -2 Give the n and values of the following orbitals. N 5 L 0.
N 3 l 1 d. Principal Quantum Number n. N 3 l 0 c.
Give the n and I values for the following orbitals. N 3 l - Brainlyph. N 1 2 3 8.
An electron located in the p subshell can reside in three p orbitals because the magnetic quantum number can take three possible values. Find step-by-step Chemistry solutions and your answer to the following textbook question. Name the orbitals described by the following quantum numbers a n 3 l 0 3s b n 3 l 1 c n 3 l 2 3d d n 5 l 0 10.
Name the orbitals described by the following quantum numbers a n 3 0b n 3 1cn 3 2d n 5 0. Represents the shape of the orbital- s p f d. Give the n and L values for the following orbitals a.

Doc Quantum Numbers Jun Bayota Academia Edu

Solved 1 Name The Orbitals Described By The Following Chegg Com

What Is The Maximum Number Of Orbitals That Can Be Identified With The Following Quantum Numbers Youtube
Solved 1 State The Four Quantum Numbers And The Possible Chegg Com

Orbitals Quantum Numbers Electron Configuration Multiple Choice Practice Problems Youtube

Quantum Number Orbital Definition Formula Diagram Shape

Magnetic Quantum Number And Shape Of Orbital Google Search Chemistry Textbook Chemistry Teaching Chemistry

Solved 1 State The Four Quantum Numbers And The Possible Chegg Com

Solved Quantum Numbers Worksheet Name 1 State The Four Chegg Com
Solved 1 Qta Name The Orbitals Described By The Following Chegg Com

Solved Name The Orbitals Described By The Following Quantum Numbers And Name One Element In That Orbital N 3 I 0 N 2 1 N 5 1 3 N 6 2 Give The N And Values Of The

Mcat Chemistry Atomic Orbitals Quantum Numbers Flashcards Quizlet

Solved Quantum Numbers Worksheet Name Ras Muinidlsti 1 Chegg Com
1 5 1 Quantum Numbers Worksheet Key

Solved State The Four Quantum Numbers And The Possible Values They May Have Name The Orbitals Described By The Following Quantum Number A N 3 0 B N 3 1 C N 3 2 D N 5 1




Comments
Post a Comment